Now, the question is whether fusion is an exothermic or endothermic process? A fusion reaction is an exothermic process as energy is released. The mass of the product formed is less than the reactants, and consequently, energy is released; therefore, the reaction is exothermic. The fusion of lighter nuclei is an exothermic process, whereas fusion of heavier nuclei is an endothermic process.
What is a Nuclear Fusion Reaction?
Nuclear fusion is defined as the reactions in which two or lighter nuclei combine, resulting in heavier nuclei and subatomic particles (neutrons or protons) with the release of a great amount of energy. The process occurs under extreme pressure and temperature conditions. In nuclear fusion, the difference between the mass of products formed and the reactants results in the release of energy. In a fusion reaction, the release of energy accounts for the interplay of nuclear force (the force that binds the protons and neutrons) and Coulomb force (the force that causes the repulsion between the protons). The sun and stars in the universe are alive due to nuclear fusion occurring in them. The reaction occurs in the core of the stars and produces a large amount of heat and energy. The fusion of hydrogen nuclei in stars resulting in the formation of helium is an example of a nuclear fusion reaction. Some of the reactions depicting nuclear fusion are given as follows: 11H + 21H → 32He (Fusion of protium and deuterium, isomers of hydrogen; to form helium) 32He + 32He → 42He + 211H (Fusion of two Helium-3 isotopes resulting in the formation of Helium-4 isotope and two protium atoms) A nuclear fusion process that results in the formation of heavier nuclei having masses greater than that of iron-56 or nickel-62 is generally endothermic. These have large masses and smaller binding energy (separation energy) per nucleon; therefore, energy is required to carry out the process, and thus it is endothermic.
What is Nuclear Fission? How is it different from fusion?
When a neutron hits a larger atom, it forces it to excite and split into two smaller atoms, which is called fission. During the process, a highly accelerated neutron bombards the heavy, unstable molecule, splitting into lighter ones. As the atom splits, a large amount of energy is released; therefore, it is an exothermic process. The release of energy is because the products formed due to fission are more stable than the reactants (more energetic). The most commonly used elements for nuclear fission reactions in reactors are uranium and plutonium. Nuclear fission can occur naturally, as in the case of radioactive metal decay, or it can be carried out in a reactor forcefully. An example of nuclear fission reaction where a highly accelerated neutron bombards with the Uranium to give Strontium and Xenon can be given as follows: 23592U + 10n → 9038Sr + 14354Xe + 310n Both fusion and fission are nuclear reactions producing enormous amounts of energy. However, the amount of energy released in nuclear fusion is several times more than that released during nuclear fission. Fission is a controlled process; hence, it finds its use in nuclear reactors, whereas fusion is not controlled and expensive to create the required environment and therefore cannot be used for the production of power. While different, the processes have an important role in energy creation.
Exothermic vs. Endothermic Reactions
In a chemical reaction, the main change occurs due to the change in bonds of molecules which happens due to the breaking of old bonds and formation of new bonds. During the formation and breakdown of bonds, energy is transferred. Energy is required to break the bonds, whereas energy is released when bond formation occurs. The chemical reactions which release energy are known as exothermic reactions. The energy released during the bond formation in a chemical reaction is more than the energy required to break the bonds of reactants. There is a significant rise in the temperature of the reaction mixture in an exothermic reaction. Similarly, the reactions which require energy to process are called endothermic reactions. In endothermic reactions, the energy required for the bond breaking of the reactants is greater than the amount of energy released in the bond formation of the products. The endothermic reactions are accompanied by the decrease in temperature of the reaction mixture. The energy released or absorbed is in the form of heat. The heat content of any system is called enthalpy. The change in enthalpy can represent the energy changes due to transfer in a chemical reaction. A reaction with a positive enthalpy change accompanies the absorption of energy. Thus, an endothermic reaction has a positive enthalpy change. While for a reaction with the release of energy, i.e., an exothermic reaction has a negative enthalpy change.
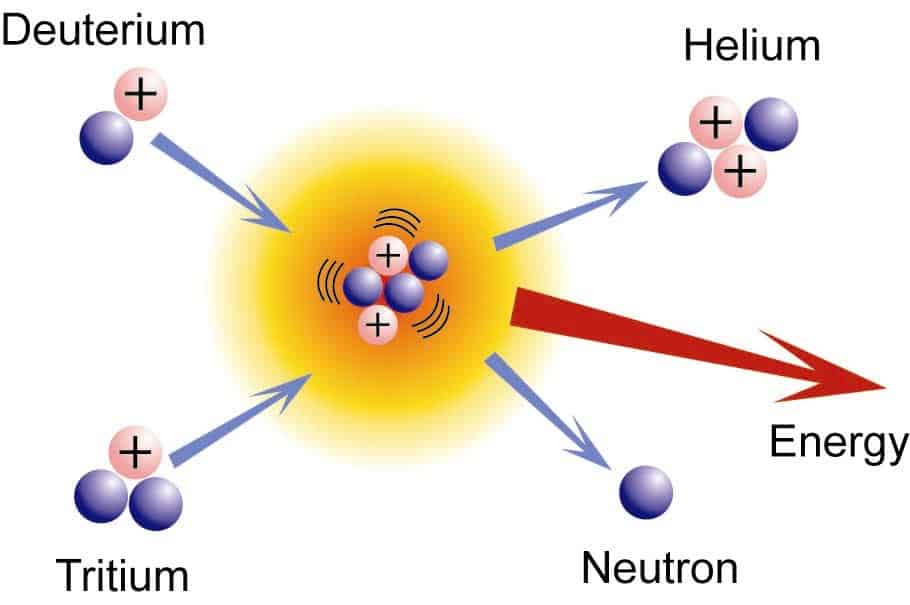
Why is Fusion an Exothermic Process?
The process of fusion involves the two lighter nuclei combining into heavier nuclei. The difference in masses of the nuclei is the reason for the energy transfer in the reaction. The mass of products formed during the process is less than the mass of the reactants. To understand in a better way how fusion is an exothermic reaction, let us consider an example of the fusion of deuterium and tritium resulting in the formation of helium nuclei. 21H + 31H → 42He + 10n The mass of the above nuclei are given as: ● neutron = 1.009 u ● deuterium nucleus = 2.014 u. ● tritium nucleus = 3.016 u ● helium nucleus = 4.003 u The difference in the masses is given as = mass of products – mass of reactants = (mass of Helium nuclei + mass of neutron) – (mass of deuterium + mass of tritium) = (4.003 + 1.009) – (2.014 + 3.016) = 5.012 – 5.030 = – 0.018 u The product nuclei have less mass than the reactant. The relation between the difference in mass given off as energy is given by the equation, E = mc2. As the differences between the masses are negative, the enthalpy obtained as per the above equation is negative. And we discussed earlier that the negative enthalpy is obtained when the reaction proceeds with the release of energy. Hence the above-discussed reaction is exothermic. This shows how fusion reactions are exothermic.
Advantages of Nuclear Fusion over Nuclear Fission
Nuclear fusion and fission reactions find their application in creating energy (or electricity). Still, the fusion process has some advantages over the latter one, which is as follows:
1. Sustainability
The fuels obtained from fusion processes are widely available and inexhaustible. Deuterium is readily extracted from seawater, while the fusion from lithium can obtain tritium. In the case of fission, Uranium has to be mined and enriched before the process and is also rare.
2. Zero radioactive waste
Nuclear fusion does not produce harmful or toxic radioactive products having a long half-life, unlike nuclear fission.
3. Safe
The amounts of fuel used for nuclear fusion are small compared to fission reactors. This is to ensure that uncontrolled releases of energy do not occur. Related posts you must read Is Sublimation Endothermic or Exothermic Is Melting Endothermic or Exothermic Is Condensation Endothermic or Exothermic
Conclusion
Nuclear fusion is a process in which lighter atoms combine to form a heavier atom and sub-particles under extreme pressure and temperature conditions with the release of enormous amounts of energy. The release of energy makes the process an exothermic reaction. In an exothermic reaction, the energy released due to bond formation in products is greater than the amount of energy absorbed while the bond breaks in the reactants, and this is accompanied by an increase in the temperature of the reaction mixture. Also, a fusion of lighter nuclei is an exothermic process, while the fusion of heavier nuclei (very few) is an endothermic process. The fusion reactions are exothermic is proved by the fact that the difference in masses of the reactants and the products is given off in the form of energy. We obtain a negative enthalpy for a fusion process, indicating that the reaction is exothermic. Fusion reactions produce more energy than fission reactions, but they have several advantages over the latter reactions. Nuclear fusion reactions are safe, clean with no radioactive waste, and sustainable compared to nuclear fission reactions. At the end of the article, we can conclude that the fusion reactions are accompanied by the release of energy, and hence fusion reactions are exothermic.